As reported from TheHeart.org:
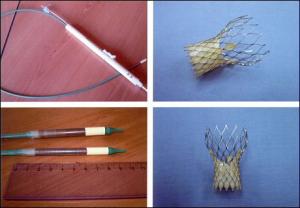
Percutaneous implantation of the self-expanding CoreValve aortic valve bioprosthesis in the first-ever series of patients shows that the procedure is feasible in those deemed too high risk for valve surgery and, when successful, results in marked hemodynamic and clinical improvement at 30 days [1].The lessons learned included:
However, there was a high periprocedural mortality of 20%, and there are several important lessons to be learned from this first-in-human single-center study, say the researchers, led by Dr Eberhard Grube (HELIOS Heart Center, Siegburg, Germany). They report their findings in the October 10, 2006 issue of Circulation.
- From TheHeart.org:
First, the periprocedural morality was high (five of the 25 patients died). Two patients died of procedure-related events (one from wire perforation of the left ventricle, one after the inability of the prosthesis to cross a heavily calcified valve). A third patient was not pretreated with clopidogrel and died of disseminated intravascular coagulation, while two others who also did not receive a thienopyridine before the implant developed a severe thrombocytopenia of prolonged duration.
- Surgery in the type of patients studied often carried operative mortalities as high as 50%
- Extracorporeal circulatory support was used in the early phase of the trial and that this could have contributed to complications (Especially thrombocytopenia - a low blood product count called platelets that can lead to severe bleeding.
- The optimal antiplatelet and antithrombotic (blood thinning) regimen to support implantation of the CoreValve device needs to be clarified
- The precise positioning of the device "remains challenging and was responsible for half the procedural failures. In this regard, the role of transesophageal echocardiography to guide placement continues to evolve, and markers are being added to the device to facilitate accurate positioning."
- Larger device sizes need to accommodate men's larger aortic sizes.
It's too early to know if this self-expanding design will supplant or complement the balloon-delivered aortic valve replacement called the Edwards Life Sciences Aortic PHV device but it is a courageous start.
-Wes
[1] Reference: Grube E, Laborde JC, Gerckens U, et al. Percutaneous implantation of the CoreValve self-expanding valve prosthesis in high-risk patients with aortic valve disease. The Siegburg first-in-man study. Circulation 2006; 114: 1616-1624.
1 comment:
I saw a presentation at ACC in March with similar data (guess it was the same researchers as this is presented as the first series of pts, don't recall the specifics). Inclusion criteria basically were those who would never be considered for AVR surgically (ie, comorbid conditions, advanced age). So, if they can improve technology as time goes on, then this may be a true option for those who had no options for their AS.
Post a Comment