While there are clearly some advantages to a implantable cardiac defibrillator that does not need to have endocardial leads, there are some disadvantages, too. Where this will leave the clinical acceptance of this new technology remains to be seen.
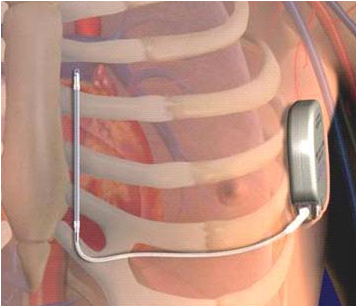 |
| The Subcutaneous ICD (S-ICD) implantation location. |
An entirely extravascular ICD system has distinct advantages for patient's with no vascular access or with pre-existing indwelling catheters that might risk infecting their permanent defibrillator leads (e.g., dialysis patients or patients receiving chemotherapeutic agents via Medi-ports or the like).
One of the bigger concerns of this technology is that patients might receive more shocks for tachyarrhythmia therapy since antitachycardia pacing is not possible with a totally extravascular subcutaneous ICD system. (Temporary backup pacing after shock is possible via the subcutaneous ICD system, however). Certainly, for clinically-indicated ICD therapy for primary prevention of sudden death in patients who do not need long-term pacing therapy, the subcutaneous ICD might find a clinical niche. But the need to increase sensitivity to low-amplitude ventricular fibrillation signals through the use of three large sensing vectors between sensing electrodes on the chest, makes the potential for sensing outside interference from electromechanical sources potentially greater with the S-ICD system compared to conventional endocardial ICD systems. In the pivotal trial presented to the FDA, it is interesting to note that there were 48 inappropriate shocks that occurred in 38 patients with the subcutaneous ICD system (30.6% of the shocks delivered). Inappropriate shock incidence in conventional ICD trials were somewhat lower (MADIT II ICD 11.5%, SCD HeFT 17%, and DEFINITE trial 21%) on my review. Strangely, the FDA executive summary of the S-ICD pivotal trial stated "FDA’s review of the literature suggests that approximately 1/3 of all shocks are inappropriate in a similarly indicated population with transvenous systems" without citing references suggesting they weren't concerned about the difference. (Am I missing something?)
Fortunately, the S-ICD's ability to appropriately defibrillate ventricular fibrillation or ventricular tachycardia appropriately was 100% in patients studied the pivotal clinical trial.
Another interesting note is that flouroscopy was not need for implantation of the subcutaneous ICD in the majority of cases. Only where defibrillation thresholds were found to be unacceptable and lead location needed to be checked what fluoroscopy used in the pivotal trial, and most of that use occurred in one center. While many of these devices may be implanted in conventional operating rooms based on anatomic landmarks, it was clear that if the device had poor defibrillation thresholds or inability to induce ventricular fibrillation to test the device, a portable flouroscopic C-arm might still be needed.
For electrophysiologists, the right device for the right patient will ultimately depend on several factors once the S-ICD is approved: (1) the systems' reliability AND longevity (there were three early battery depletions in the pivotal trial), (2) the technical support provided, (3) the clinical indication for the ICD, (4) physician acceptance of the technology (5) the patient co-morbidities, and to some extent, (6) cost. My bet is that secondary prevention patients (e.g., those who have already had a ventricular tachyarrhythmia) and patients still in need of pacing (conventional or biventricular) will tend to continue to receive endovascular ICD devices. Patients with several congenital disorders (HCM, Long QT, Brugada, etc.) or indwelling catheters will be more likely to receive subcutaneous ICD devices in the years ahead, provided they do not have a significant arrhythmia burden.
The problem is, doctors can't predict the arrhythmia type or arrhythmia burden that might develop ahead of time. Subcutaneous ICD therapy greatest asset, simplicity, is also it's greatest limitation long-term. The device simply can't adapt its therapies to changes in clinical situations (or arrhythmias) that develop later. As such, the widespread acceptance of this technology still has a ways to go, but its niche market remains, making it a valuable addition to the EP's options for life-saving therapy for our patients.
-Wes
Reference:
FDA Executive Summary P110042 Subcutaneous Implantable Cardioverter Defibrillator (S-ICD) System, Cameron Health, Inc. April 26, 2012.
1 comment:
Good points.
Another disadvantage of the Subcutaneous ICD is the fact that the device does not have remote monitoring capabilities, at least not yet. And that's a big one in my view.
Post a Comment