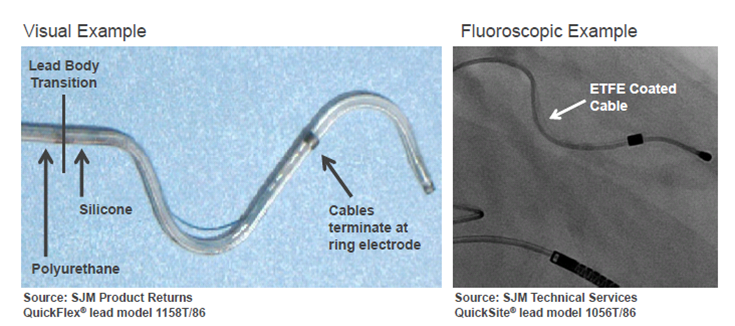
 |
| (Click image to enlarge) |
For St. Jude Medical, this latest revelation comes at a difficult time as physicians are still trying to deal with the management of St.Jude's earlier-generation recalled silicone defibrillator leads, the Riata and Riata ST. The revelation that this externalization can now affect St. Jude's bradycardia left ventricular pacing leads will no doubt affect physician's device selection. It is also clear that St. Jude Medical's portfolio of products for their lucrative cardiac rhythm management division will be significantly impacted.
For patients with these left ventricular pacing leads, follow-up of their system may involve x-ray evaluation on an intermittent basis, but I do not think that St. Jude and its physician advisory board will recommend removal of these leads, given the risks of infection and trauma that might be inherent to that procedure.
More likely, careful routine follow-up of these leads will be all that is required for now with the addition of at least one surveillance x-ray evaluation of the patient's LV lead and RV lead systems.
-Wes
4 Apr 2012 @ 10:55 am CST: Updated to include photo.
Disclaimer: Please understand that the opinions expressed in this blog are mine alone and do not represent the views of St. Jude Medical nor NorthShore University HealthSystem. I have no financial ties with St. Jude Medical but do serve on the speaking circuits for Medtronic, Boston Scientific, and Zoll Medical.
4 comments:
Do you think an X ray will be enough to detect that subtle externalization in a small LV lead? Maybe routine fluoroscopy is indicated.. also, I don't understand how pacing impedance is not affected, could you explain this?
mcg-
The use of fluoro or xray is controversial as a screening procedure with this problem and whether ANYTHING will need to be done outside of conventional follow-up remains to be seen. However, imaging (by way of fluoro or careful xray exam) may be the only way we can determine the full extent of this problem for our patients clinically. The externalization of the wire may be subtle to detect irrespective of imaging modality used.
The wire itself has an separate insulation layer over it (even when externalized from the main body of the lead) and this might be why abnormal impedances have not been observed with this defect so far.
Wes,
This information is really early and treatment strategies are yet to be worked out.
That being said, I doubt I'll be doing routine fluoro exams for this lead.
The electrical function of this lead is straightforward to assess with standard testing. Unless externalization is associated with dysfunction, it may not have much significance.
I'm not sure knowing a LV has externalized would affect management strategies to any degree. I might be useful to do fluoro as part of a clinical trial, though, to help determine it's significance.
Even routine fluoro or Riata/Riata ST is of uncertain benefit, and that's a case in which there may be more potential utility.
Thanks for reporting on this important development.
Jay
@EJSMD
This failure mechanism may be of significance should the dysfunction be associated with oversensing from the LV lead itself.
In this era of mix and match, there are/could be patients out there with BSX devices connected to these SJM LV leads.
BSX being one of the only companies employing LV sensing in their devices, noise/oversensing on an SJM QuickSite or QuickFlex LV lead would trigger the 'Noise Response' feature of these devices.
The Cognis CRT-D System Guide states the following:
If noise inhibits in LV Only, the device will issue an RV pace for bradycardia support.
Certainly the presence of noise on an LV lead wouldn't result in asystole in a dependent patient; but it could result in extended periods of RV only pacing in heart failure patients in deperate need of true biventricular pacing.
@neillreynolds
Post a Comment